By Dr. Kingsley Chin
Scientific Paper
Interested medical professionals can read through the full paper, also published in PLOS One, here.
Background
The neurohypophysial hormone arginine vasopressin (AVP) plays important roles in fluid regulation and vascular resistance. Differences in AVP receptor expression, particularly mediated through variation in the noncoding promoter region of the primary receptor for AVP (AVPR1a), may play a role in social phenotypes, particularly social monogamy, in rodents and humans. Among primates, social monogamy is rare, but is common among New World monkeys (NWM). AVP is a nonapeptide and generally conserved among eutherian mammals, although a recent paper demonstrated that some NWM species possess a novel form of the related neuropeptide hormone, oxytocin. We therefore characterized variation in the AVP and AVPR1a genes in 22 species representing every genus in the three major platyrrhine families (Cebidae, Atelidae and Pitheciidae). For AVP, a total of 16 synonymous substitutions were detected in 15 NWM species. No non-synonymous substitutions were noted, hence, AVP is conserved in NWM. By contrast, relative to the human AVPR1a, 66 predicted amino acids (AA) substitutions were identified in NWM. The AVPR1a N-terminus (ligand binding domain), third intracellular (G-protein binding domain), and C-terminus were variable among species. Complex evolution of AVPR1a is also apparent in NWM. A molecular phylogenetic tree inferred from AVPR1a coding sequences revealed some consensus taxonomic separation by families, but also a mixed group composed of genera from all three families. The overall dN/dS ratio of AVPR1a was 0.11, but signals of positive selection in distinct AVPR1a regions were observed, including the N-terminus, in which we identified six potential positive selection sites. AA substitutions at positions 241, 319, 399 and 409 occurred uniquely in marmosets and tamarins. Our results enhance the appreciation of genetic diversity in the mammalian AVP/AVPR1a system, and set the stage for molecular modeling of the neurohypophyseal hormones and social behavior in primates.
Arginine vasopressin (AVP) coding sequences for primates.
New World monkeys (NWM) are indicated by shaded area. ‘.’ represents identity with the human AVPR1a sequence.
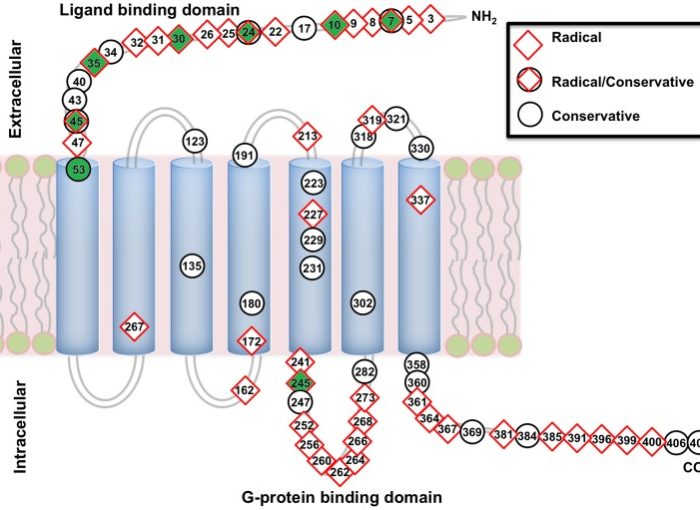
Structural model of AVPR1a.
Numbers reflect amino acid substitutions in NWM relative to the human AVPR1a, and radical physicochemical substitutions are indicated by red diamonds and conservative changes by black circles. Potential positively selected sites are highlighted in green.
Molecular phylogenetic trees in primates.
A. Tree inferred from AVPR1a nucleotide coding sequences in primates. If bootstrap support is <60, no value is shown at nodes. Scale bar indicates the branch length in nucleotide substitutions per site. A red diamond indicates a genus characterized by social monogamy. B. A consensus tree of primates based on 54 nuclear genes (34,927 bp; [30]). Families Cebidae, Atelidae and Pitheciidae are highlighted in green, red and purple lines, respectively. Amino acid substitutions of the AVPR1a gene are plotted on the consensus tree: NWM-specific (green square), Callitrichinae-specific (red square), marmoset-specific (blue square), marmoset and Callicebus-specific (yellow square), and genera-specific (black square).
Sliding window analysis of dN/dS ratios and dN values along the NWM AVPR1a.
The ratio/value are drawn over the midpoint window position (window length 50, step size 10) from whole coding region. A dN/dS ratio above 1 indicates possible positive selection in the region. The elements of AVPR1a are highlighted in red (extracellular), yellow (transmembrane), and green (intracellular) lines.
About Author Dr. Kingsley R. Chin
Dr. Kingsley R. Chin, Founder of philosophy and practice of The LES Society and The LESS Institute
Dr. Kingsley R. Chin is a board-certified Harvard-trained Orthopedic Spine Surgeon and Professor with copious business and information technology exposure. He sees a niche opportunity where medicine, business and info. tech meet – and is uniquely educated at the intersection of these three professions. He has experience as Professor of Clinical Biomedical Sciences & Admissions Committee Member at the Charles E. Schmidt College of Medicine at Florida Atlantic University, Professor of Clinical Orthopedic Surgery at the Herbert Wertheim College of Medicine at Florida International University, Assistant Professor of Orthopaedics at the University of Pennsylvania Medical School, Visiting Spine Surgeon & Professor at the University of the West Indies, Mona, and Adjunct Professor of Clinical Biomedical Sciences at the University of Technology, Jamaica.
Learn more about Dr. Chin here and connect via LinkedIn.
About Less Exposure Surgery
Less Exposure Surgery (LES) is based on a new philosophy of performing surgery, leading the charge to prove through bench and clinical outcomes research that LES treatment options are the best solutions – to lowering the cost of healthcare, improving outcomes and increasing patient satisfaction. Learn more at LESSociety.org.
The LES Society philosophy: “Tailor treatment to the individual aiding in the quickest recovery and return to a pain-free lifestyle, using LES® techniques that lessen exposure, preserve unoffending anatomy and utilize new technologies which are safe, easy to adopt and reproducible. These LES®techniques lessen blood loss, surgical time and exposure to radiation and can be safely performed in an outpatient center. Less is more.” – Kingsley R. Chin, MD
About The LESS Institute
The LESS Institute is the world leader center of excellence in Less Exposure Surgery. Our safe, effective outpatient treatments help patients recover quickly, avoid expensive hospital stays and return home to their family the same day. Watch our patient stories, follow us on Facebook and visit TheLESSInstitute.com to learn more.
About SpineFrontier
The above study utilized LES Technology from SpineFrontier – leading provider of LES Technologies and instruments – offering surgeons and patients superior technology and services.
Scientific Paper Author and Citation Details
Authors
Author information
Callitrichid Research Center, Department of Psychology, University of Nebraska at Omaha, Omaha, Nebraska, United States of America; Key Laboratory for Animal Biotechnology of Jiangxi Province and the Ministry of Agriculture of China, Jiangxi Agricultural University, Nanchang, China; Department of Biology, University of Nebraska at Omaha, Omaha, Nebraska, United States of America.
Callitrichid Research Center, Department of Psychology, University of Nebraska at Omaha, Omaha, Nebraska, United States of America; Department of Biology, University of Nebraska at Omaha, Omaha, Nebraska, United States of America.